Physical Science Student Work

Vision for Student Work Page:
During my teaching in Alcona, I kept a "running log" of some of the activities and labs we do in class. Below are six different labs that we did in General Science. I would like this space to be a spot where students can look back at all of their accomplishments of the course!
Waves Lab:
This lab was used as an introduction to waves. The students had six different stations to investigate waves. The pictures above show the Tuning Fork Station, Marble Station, Coffee Can Station and Water Station. Not pictured are the Slinky Station and Rope Station. In each station the students had to make different kinds of "waves" and describe or draw the waves they made in each station. Through out the rest of the unit, we came back to these labs to describe the different kinds of waves and their different properties.
Density:
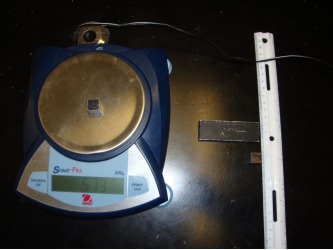
In the picture on the left, students found the density of three different objects, two identical looking cubes and a slab. Students had to find the mass and volume of each in order to find their density. They then compared the density of each object to see if the objects were made of the same substance or different substances. One cube and the slab were both made of aluminum and the other cube was made of iron.
In the picture on the right, students had to compare two unknown liquids. They did this by again finding the density of each of the liquids. The two liquids were both salt and water solutions, but with different concentrations. Thus, the density of each would be different showing that A and B were indeed different substances.
Density is a characteristic property. Characteristic properties are properties that are unique to a substance, and can be used to identify a substance.
Freezing and Melting:
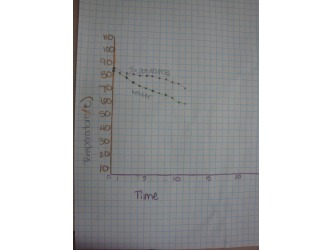
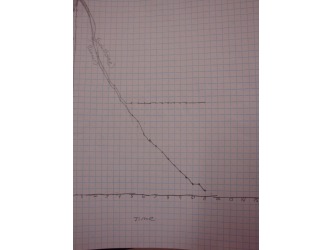
Students heated up one of two substances (TOP or BHT) in a water bath until the solid substance became a liquid. Then watched and recorded the temperatures of the substance and the water as each cooled down. When they graphed their recorded temperatures, students were able to see that as a substance is changing state (in this case the substance is freezing) it remains at a constant temperature. This is another characteristic property. After this lab they were able to add Melting and Boiling points to their list of characteristic properties.
The two pictures on the right are examples of graphs students made to compare the temperatures of the substance and the water. These clearly show that the substance remains at the same temperature when it is changing state.
Solubility and Concentrations:
Above shows two labs in which students investigated the solubility of solutions. In the two on the left, students found the concentration of a saturated solution. They first began with 5 g of either Sodium Nitrate or Sodium Chloride (they did not know which one they had) and added 5 ml of water to it, shaking up the solution until it became saturated. Pouring out only the saturated solution, not the undissolved solid at the bottom, they found the mass of the solution. They then heated up the solution to allow the water to evaporate, thus being able to find the mass of the solid in the solution. Finally the students were able to find the concentration by dividing the mass of the solid by the volume of the water that evaporated. Those with substance A and B found that they had different concentrations from each other. The solubility of a substance is anothe characteristic property!
In the picture on the right, students investigated how changing the temperature of a solution can change its solubility. They took two different saturated solutions of Sodium Chloride and Potassium Nitrate with some undissolved solid in the test tube and heated them unit the water they were in boiled. As they were heating the two solutions up, they continually stirred the solutions and watched the undissolved solid at the bottom of each. They were able to see that as the temperature increased, the amount of solid at the bottom of the Potassium Nitrate solution was decreasing, where as the Sodium Chloride undissolved solid was changing very little. This showed that as temperature increases, solubility increases, but the change in solubility is yet another characteristic property. The Potassium Nitrate's solubility increases dramatically as the temperature increases, where as the Sodium Chloride's solubility only increases slightly.